NCERT Science Solutions for Class 8 – Chapter 14 – Chemical Effects of Electric Current
Chemical effects of electric current
NCERT Solutions for Class 8 Science Chapter 14 are given below.
Question 1. Fill in the blanks.
a) Most liquids that conduct electricity are solutions of…………………., ……………….. and ………………….
Answer: acids, bases and salts
b) The passage of an electric current through a solution causes ……………………. effects.
Answer: chemical
c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ……………………. terminal of the battery.
Answer: negative
d) The process of depositing a layer of any desired metal on another material by means of electricity is called ………………….
Answer: electroplating
Question 2. When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Answer:
The deflection of the magnetic needle clearly shows that current is passing through the circuit. It also shows that the given solution is a conductor of electricity because the circuit of the tester will only become complete if the liquid between the two ends of the tester allows the electric current to pass.
Question 3. Name three liquids which when tested in the manner shown in the picture may cause the magnetic needle to deflect.
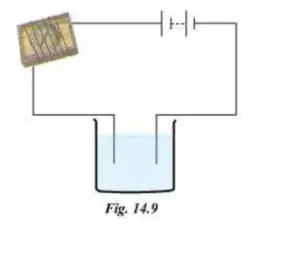
Answer:
The magnetic needle will show deflection when you dip the two ends of the tester in lemon juice, salt water and vegetable oil. These are good conductors of electricity. Tap water is also a good conductor of electricity because it normally contains small amounts of mineral salts in it.
Question 4. The bulb does not glow in the setup shown in figure 14.10. List the possible reasons. Explain your answer.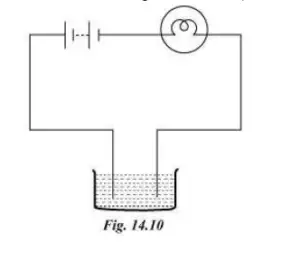
Answer:
The bulb may not glow because of the following reasons.
The bulb will not glow if the current passing through the circuit is too weak. Weak current cannot heat the filament sufficiently to produce glowing light.
The solution could be non-conducting. In this case the current cannot pass through the liquid between the two ends of the tester.
Other possible reasons include loose connections, fused bulb or used up battery.
Question 5. A tester is used to check the conduction of electricity through two liquids labeled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that:
(i) liquid A is a better conductor than liquid B
(ii) liquid B is a better conductor than liquid A
(iii) both liquids are equally conducting
(iv) conducting properties of liquids cannot be compared in this manner.
Answer:
Liquid A is a better conductor than liquid B.
Since the bulb glows brightly, it shows that the current passing through the circuit of the tester immersed in liquid A is strong. If liquid A was a poor conductor, the current would be weak and the bulb would not glow brightly.
Question 6. Does pure water conduct electricity? If not, what can we do to make it more conductive?
Answer:
No. Pure water is free of salts and does not conduct electricity. However, we can increase its conductivity by adding a pinch of common salt to it. When salt is added to pure water, it becomes a salt solution which conducts electricity.
Question 7. In case of a fire, before firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer
Tap water is usually a good conductor of electricity because it contains some amounts of mineral salts. If this water is poured over electrical appliances or live wires, the firemen will get an electric shock. To avoid this they shut off the electrical supply for the area.
Question 8. A child staying in the coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer:
Drinking water contains only a small amount of mineral salts and hence it is not a good conductor of electricity. Because of this reason when the tester is dipped in drinking water, the needle does not deflect much.
Sea water contains huge amounts of dissolved salts and hence it is a good conductor of electricity. That’s why the needle shows good deflection.
Question 9. Is it safe for the electrician to carry out repairs outdoors during heavy downpour? Explain.
Answer:
No. Rainwater may conduct electricity. So, if an electrician carries out electrical repairs outdoors during heavy rains, they run the risk of getting electrocuted.
Question 10. Paheli had heard that rainwater is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Answer
Rainwater contains dissolved salts and because of this reason it is a conductor of electricity. That is why the needle shows deflection when the tester is immersed in rainwater. Distilled water does not contain any dissolved salts and hence it is non-conducting.
Question 11. Prepare a list of objects around you that are electroplated.
Answer
Many objects like bathroom taps, gas burners, car parts, bicycle handlebars have chromium plating.
Gold plated ornaments have a thin layer of gold on less expensive metals.
Tin cans used for storing food are made by electroplating tin onto iron.
Iron used for construction purposes are coated with a layer of sink to protect it from corrosion and rusting.
Question 12. The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
Answer
The thick rod of impure copper should be attached to the positive terminal and the thin plate of pure copper should be attached to the negative terminal.
When electric current passes through the copper sulphate solution, copper gets dissociated into copper and sulphate. The free copper gets deposited on the electrode connected to the negative terminal of the battery. At the same time, an equal amount of copper from the thick impure road gets dissolved in the solution. This way copper can be removed from the impure rod and added to the pure rod.